Cancer Microbiome research,
Meyer Cancer Center (MCC) at Weill Cornell Medicine + Englander Institute for Precision Medicine (EIPM)
New York, NY, 2024 - 2025
As part of this internship, I completed a focused literature review on the role of the gut microbiome in cancer immunotherapy, with particular emphasis on CTLA-4 checkpoint blockade treatments such as ipilimumab (Yervoy). My research analyzed and synthesized findings from human and animal studies to examine how microbiome composition influences patient response, toxicity, and treatment efficacy across cancer types, especially melanoma. This work strengthened my ability to critically analyze scientific literature and connect research findings to clinical decision-making.
Alongside this research, I participated in seminars and didactic sessions on cancer biology, precision medicine, health disparities, and computational biomedicine, and worked closely with faculty and researchers across MCC and EIPM. This experience helped me translate research findings into clinical care.
The program concluded with the preparation of a formal research paper written according to NIH grant guidelines, included below.
A Literature Review of Cancer Microbiome Studies in CTLA-4 Blockade Immunotherapy
Background / Problem
Immunotherapy uses the body’s own immune system to combat cancer. It can either stimulate the natural defenses of the immune system to fight cancer or involve man-made therapies modified in the lab to enhance its ability to target cancer cells. There are multiple types of immunotherapies, including immune checkpoint inhibitors (ICIs). ICIs block checkpoint proteins from binding with their partner proteins and help signal T cells to kill cancer cells.
ICIs have transformed immunotherapy, offering a new form of treatment for cancer patients. However, as multiple studies have shown, they may not work for everyone and can have adverse side effects. The most common side effects include rash, diarrhea, fatigue, and inflammation, which can lead to complications depending on the location of the inflammation, such as pain, muscle weakness, and skin discoloration. As a result, while immunotherapy is an effective treatment for cancer, the responsiveness and effectiveness of ICIs vary across cancer types and patient populations.
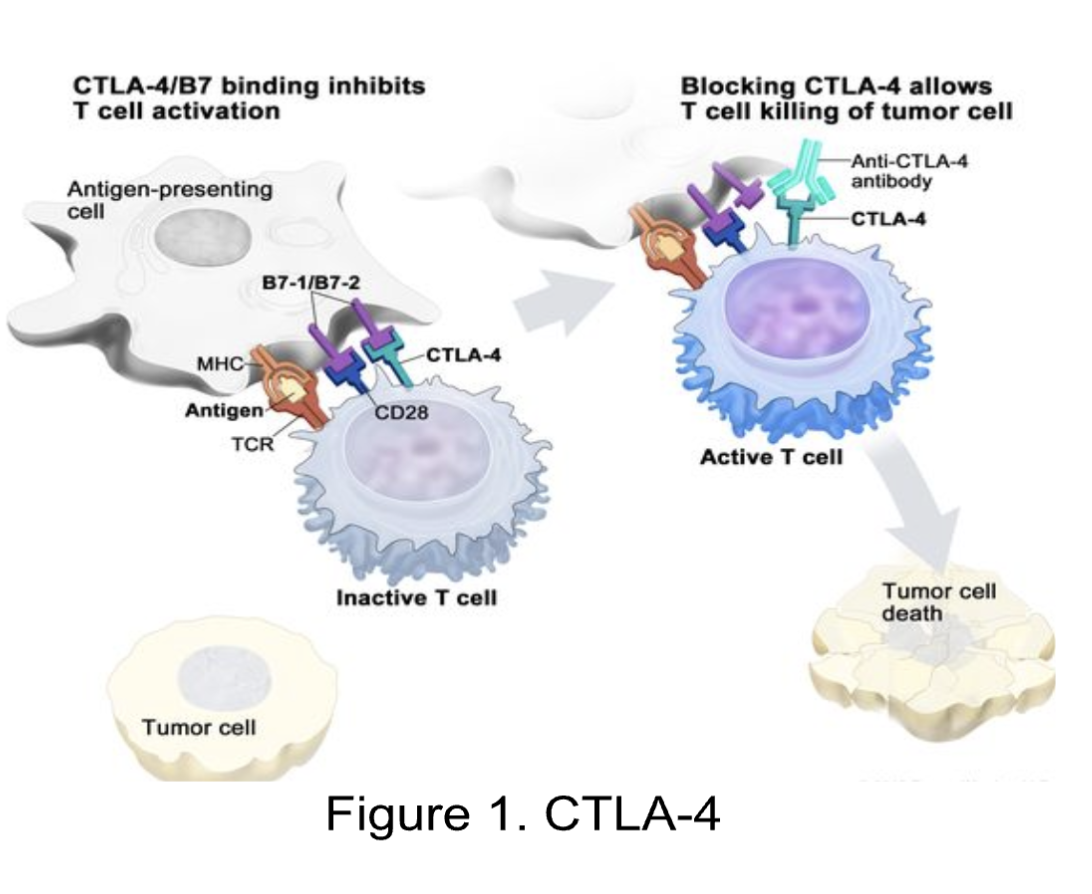
Among the most promising checkpoints, CTLA-4 is a checkpoint protein found on T cells that aids immune responses. However, when it binds to B7 (another protein), it blocks the body’s ability to kill harmful cells (Fig. 1). The CTLA-4 pathway allows tumors to evade the immune system. By blocking this pathway, the immune response against tumors is activated. Modulating the microbiota can make checkpoint blockade approaches targeting CTLA-4 more efficient.
Among these, Yervoy, or Ipilimumab, is a monoclonal antibody medication specifically targeting CTLA-4. It has significantly improved the survival of patients with advanced-stage melanoma. The discovery of Ipilimumab followed the introduction of the concept of “checkpoint blockade.” Originally, anti-CTLA-4 was successful only in mice, which posed a challenge for many scientists. Progress was made in adapting anti-CTLA-4 for human use, and the development was taken over by Bristol Myers Squibb, which named it Yervoy. Yervoy has transformed cancer immunotherapy and influenced the development of similar anti-cancer antibodies.
Predictive factors have been used to improve responsiveness, including tumor mutational burden and clinical features. Among these, the gut microbiota has recently been shown to influence the effectiveness of immunotherapy, as it plays a significant role in boosting the immune response. Since there are both commensal and pathogenic bacteria, the microbiome’s influence on treatment response is highly variable.
Objective / Hypothesis
Many studies have investigated the link between the cancer microbiome and immunotherapy, providing extensive information on the topic. Despite the volume of literature, there is a lack of consistent knowledge and structure. This makes it harder for scientists to gain a clear understanding of current research. We hypothesize that by conducting a literature review on the cancer microbiome and immunotherapy and summarizing existing knowledge and studies, we will be better prepared to study the cancer microbiome in a highly structured manner. This will allow researchers to understand the topic holistically, improving both future cancer microbiome research and the efficacy and application of cancer immunotherapy
Methods and Results
We conducted a literature review by searching PubMed with the keywords ‘cancer microbiome,’ ‘immunotherapy,’ and ‘CTLA-4.’ We identified 11 publications from 2014 to 2024 and gathered key points from these studies, including the total number of patients, patient responsiveness, microbiome data type used, cancer type, sequencing technology, and experimental validation (if any). These 11 papers ranged from mouse studies to human studies and were published in journals such as The Lancet, Nature Medicine, and Science.
We found that the highest number of samples was 77 and the lowest was 10. The most common cancer type in these studies was melanoma, which appeared in 6 of the 11 studies. Other cancer types included renal cell carcinoma, colorectal cancer, and pancreatic cancer. All studies reported responsiveness to anti-CTLA-4, as well as anti-PD-1 or anti-PD-L1. The sequencing technologies used were primarily 16S, but also included WMS and qPCR. Most studies included experimental validation as a follow-up investigation.
Future Directions
Despite reaching a strong conclusion from our research, there were limitations to the published studies. Each publication on CTLA-4 had a small sample size, which limited the tests. Most studies are from the US, leading to results based on a less diverse population. CTLA-4 remains more understudied compared to PD-1/PD-L1, resulting in less data and research available for scientists. Microbiome studies primarily use 16S sequencing, which does not produce high-resolution data, making some results more inconclusive.
For the future of CTLA-4 research, larger studies across a broader range of cancer types are needed to fully understand the potential impact of the gut microbiome on cancer immunotherapy. Including diverse populations will enhance the understanding of possible influences on results. Meta-analysis can improve current discoveries and reveal patterns within the research. These future steps could significantly advance our knowledge of the cancer microbiome and its relationship to immunotherapy.
Works Cited
Andrews, M. C., Duong, C. P. M., Gopalakrishnan, V., Iebba, V., Chen, W.-S., Derosa, L., Khan, M. A. W., Cogdill, A. P., White, M. G., Wong, M. C., Ferrere, G., Fluckiger, A., Roberti, M. P., Opolon, P., Alou, M. T., Yonekura, S., Roh, W., Spencer, C. N., Curbelo, I. F., . . . Cooper, Z. A. (2021). Gut microbiota signatures are associated with toxicity to combined ctla-4 and pd-1 blockade. Nature Medicine, 27(8), 1432-1441. https://doi.org/10.1038/s41591-021-01406-6
CTLA-4. (n.d.). National Cancer Institute. Retrieved August 6, 2024, from http://Immune Checkpoint Inhibitors
Fessler, J., Matson, V., & Gajewski, T. (2019). Exploring the emerging role of the microbiome in cancer immunotherapy. Springer Link, 7(108). https://link.springer.com/article/10.1186/s40425-019-0574-4
Immune Checkpoint Inhibitors. (n.d.). National Cancer Institute. Retrieved August 6, 2024, from https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/checkpoint-inhibitors
Joachim, L., Göttert, S., Sax, A., Steiger, K., Neuhaus, K., Heinrich, P., Fan, K., Orberg, E. T., Kleigrewe, K., Ruland, J., Bassermann, F., Herr, W., Posch, C., Heidegger, S., & Poeck, H. (2023). The microbial metabolite desamino tyrosine enhances t-cell priming and cancer immunotherapy with immune checkpoint inhibitors. EBioMedicine, 97, 104834. https://doi.org/10.1016/j.ebiom.2023.104834
Mager, L., Burkhard, R., & Mccoy, K. (n.d.). Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. https://doi.org/10.1126/science.abc3421
Ninkov, M., Schmerk, C. L., Moradizadeh, M., Parvathy, S. N., Figueredo, R., Burton, J. P., Silverman, M. S., Fernandes, R., Maleki Vareki, S., & Haeryfar, S. M. M. (2022). Improved MAIT cell functions following fecal microbiota transplantation for metastatic renal cell carcinoma. Cancer Immunology, Immunotherapy, 72(5), 1247-1260. https://doi.org/10.1007/s00262-022-03329-8
Park, H.-J., Boo, S., Park, I., Shin, M. S., Homma, K., Takahashi, T., & Takanari, J. (2022). AHCC®, a Standardized Extract of Cultured Lentinula Edodes Mycelia, Promotes the Anti-Tumor Effect of Dual Immune Checkpoint Blockade Effect in Murine Colon Cancer. Frontiers, 13. https://doi.org/10.3389/fimmu.2022.875872
Roviello, G., Iannone, L. F., Bersanelli, M., Mini, E., & Catalano, M. (2022). The gut microbiome and efficacy of cancer immunotherapy. Pharmacology & Therapeutics, 231, 107973. https://doi.org/10.1016/j.pharmthera.2021.107973
Shin, D., Basak, S., Veena, M., & Bhattacharya, A. (n.d.). Enhanced CTLA-4 blockade anti-tumor immunity with APG-157 combination in a murine head and neck cancer. Wiley. https://doi.org/10.1002/cam4.7212
The Story of Yervoy (Ipilimumab). (n.d.). Cancer Research Laboratory. Retrieved August 6, 2024, from https://crl.berkeley.edu/discoveries/the-story-of-yervoy-ipilimumab/
Tsakmaklis, A., Farowski, F., Zenner, R., Lesker, T. R., Strowig, T., Schlößer, H., Lehmann, J., von Bergwelt-Baildon, M., Mauch, C., Schlaak, M., Knuever, J., Schweinsberg, V., Heinzerling, L. M., & Vehreschild, M. J. G. T. (2023). TIGIT+ NK cells in combination with specific gut microbiota features predict response to checkpoint inhibitor therapy in melanoma patients. BMC Cancer, 23(1). https://doi.org/10.1186/s12885-023-11551-5
Ueki, H., Kitagawa, K., Kato, M., Yanase, S., Okamura, Y., Bando, Y., Hara, T., Terakawa, T., Furukawa, J., Nakano, Y., Fujisawa, M., & Shirakawa, T. (2023). An oral cancer vaccine using bifidobacterium vector augments combination of anti-PD-1 and anti-CTLA-4 antibodies in mouse renal cell carcinoma model. Scientific Reports, 13(1). https://doi.org/10.1038/s41598-023-37234-6
Vetizou, M., Pitt, J., & Daillere, R. (2015). Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science, 350(6264). https://doi.org/10.1126/science.aad1329
Vryza, P., Fischer, T., & Mistakidi, E. (2023). Tumor mutation burden in the prognosis and response of lung cancer patients to immune-checkpoint inhibition therapies. Science Direct, 38. https://doi.org/10.1016/j.tranon.2023.101788
Zhang, B., Li, H., Liu, J., Huang, B., & Zhang, B. (2023). Integrated multi-omics identified the novel intratumor microbiome-derived subtypes and signature to predict the outcome, tumor microenvironment heterogeneity, and immunotherapy response for pancreatic cancer patients. Frontiers, 14. https://doi.org/10.3389/fphar.2023.1244752
Zitvogel, L., Ma, Y., Raoult, D., Kroemer, G., & Gajewski, T. F. (2018). The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science, 359(6382), 1366-1370. https://doi.org/10.1126/science.aar6918